Research Article - Journal of Environmental and Occupational Health (2022)
Risk Status of Malaria Based on Sociodemographic, Behavioural and Environmental Risk Factors in Two Communities in Lagos, Nigeria
Abdulrahman Babaatunde Bello* and Adesola Adebolade HassanAbdulrahman Babaatunde Bello, Department of Zoology, University of Ibadan, Ibadan, Nigeria, Email: bellobabatunde9@gmail.com
Received: 28-Feb-2022, Manuscript No. JENVOH-22-55730; Editor assigned: 02-Mar-2022, Pre QC No. JENVOH-22-55730 (PQ); Reviewed: 17-Mar-2022, QC No. JENVOH-22-55730; Revised: 22-Mar-2022, Manuscript No. JENVOH-22-55730 (R); Published: 29-Mar-2022
Abstract
Background: Studies highlighting the influence of sociodemographic, cultural and environmental factors on malaria incidence have been able to elucidate the risk status of malaria. The sociodemographic, cultural and environmental risk variables influencing malaria occurrence was evaluated.
Methods: Blood samples were collected from 360 multistage systematically selected household heads in Epe and Orimedu. Pretested and structured questionnaires were administered to the participants to obtain data related to their sociodemography, mosquito prevention practices and environmental factors of vulnerability to malaria. Potential risk factors for the occurrence of malaria were analyzed using binary logistic regressfion (Odds Rafio: OR). Chfi-Square (X2) was used to analyze the relationship between malaria parasite test outcome and bed net ownership, standard mosquito prevention practices awareness, presence of surrounding stagnant pools and possession of window/door nets.
Results: The odds of having malaria was relatively high among participants aged 60 years and above, OR=1.35, C.I (0.29-6.21), pensioners, OR=3.00, C.I (0.19-47.96), participants without previous malaria episode since the last one year, OR=1.33, C.I (0.86-2.08) and participants who are not aware of malaria, OR=2.11, C.I (0.13-34.08). Low odds of having malaria was observed among participants: who sought treatment in health facilities, OR=0.00, C.I (0.00) and who knew that malaria can be prevented, OR=0.54, C.I (0.20-1.45). Malaria prevalence was high among participants who own bed nets (2(3) =1.81, p>0.05), have windows/door nets (2(3) =2.33, p>0.05) and who do not have surroundings stagnant pools (2(3) =1.17, p>0.05).
Conclusion: Age, educational level, occupation, previous malaria episodes, treatment facility of choice, malaria awareness and knowledge about mosquito prevention practices influence malaria risk status. However, bed net ownership, possession of window/door nets and absence of surrounding puddles do not significantly reduce malaria incidence.
Keywords
Sociodemographic factors; Malaria infection occurrence; Environment; Risk status of malaria
Introduction
Malaria infection occurrence is a function of the sociodemographic, cultural, behavioural and environmental conditions in a particular geographic area [1,2]. These factors influence risk status of malaria, level of susceptibility, social interaction as well as behaviours, thereby promoting the disease occurrence by creating favourable environment for the malaria vectors to thrive [3]. The concept of human ecology of disease has since been a principal constituent in geographic approach to study the predisposing factors towards disease in human society [4,3,5]. The human ecology entails the various manners in which human behaviour in its sociodemographic and cultural perspectives interact with the environment to promote or hinder the occurrence of malaria among susceptible host.
The socio-economic status, housing conditions, educational level has significant impact on the risk status of malaria. For instance, people living in close proximity, about 450 m away from vector breeding sites, incessant or recent migration to malaria endemic regions, weedy surroundings, houses without ceilings, houses with a separate kitchen building, living within 200 m of a maize field and household in which the heads had no formal education are all at an increased risk of malaria infection in the highlands [3,5]. The relationships that exist between urbanization and status and the prevalence of malaria cannot be over-emphasized [6].
The chances of man to become infected with malaria mainly depend on how his behavioural traits influence the degree of human-vector contact [7]. Human occupation, farming practices, socio-demographic factors, all have strong influence on the transmission of malaria [8,9]. For instance, the geographic location and type of human habitation in relation to the availability of vector breeding habitats, night migration in malaria endemic regions, outdoor sleeping and occupation such as night guards, level of compliance to the implementation of available malaria intervention programs, such as sleeping under the LLINs, all have influence on the presence or absence of malaria infections [7].
The body mass of a host may also affect malaria risk status by influencing host selection, with a larger host tending to exude a higher quantity of olfactory cues thereby increasing the attractiveness of the vector to human. A study reported that the production of metabolic carbon dioxide is positively associated with body size [10], which may in turn influence host selection by the vector. According to [11], young children are less frequently bitten by mosquitoes compared with adult parents, due to mosquitoes expressing different degrees of preferences for humans in terms of odour profile, age, gender, among others.
Since malaria risk status has been established to vary from one geographic region to another, and is dependent on the prevailing sociocultural, economic, demographic and environmental risk variables, adoption of targeted allocation of malaria intervention strategy, in order to ensure that interventions get to the population who are at relatively increased risk of the disease will ensure successful intervention programmes. However, a good knowledge of the Sociodemographic Behavioural and Environmental (SBE) risk variables influencing the risk of malaria occurrence is prerequisite to planning targeted allocation of malaria intervention. The study therefore evaluated the SBE risk variables influencing the risk of malaria infection occurrence.
Materials and Methods
The study was conducted in Epe town, Epe Local Government Areas and Orimedu constituency, Ibeju-Lekki LGA of Lagos State, Nigeria. The numerous water bodies in Epe justify the popular fishing and other farming activities attributed to the town, being one of the major food sources, (especially fish) of the State [12]. The permanent water bodies present, which are of varying size, turbidity, depth and speed of water flow, in the area are the source of the numerous wetlands present in that town [13]. These wetlands create favourable habitats for malaria vectors to thrive all year round but with varying intensities. The human settlements that are close to these wetlands may likely experience an almost all round the year breeding of mosquitoes [14], and subsequent malaria transmission [15]. Malaria transmission in Orimedu also occurs throughout the year with peaks occurring in the rainy season [15].
The study is a descriptive cross sectional survey and experimental design, which consists of parasitological and environmental studies. Ethical approvals were obtained from the Lagos State University Teaching Hospital (LSUTH) ethical review board (Reference No: LREC/06/10/1315) and University of Ibadan/ University College Hospital (UI/UCH) Ethics Committee, IAMRAT, College of Medicine, University of Ibadan (Reference No: UI/EC/19/0523). Permissions were obtained from relevant authorities and participants’ informed consents were also obtained. A sample size of 360, which is shared equally among the 2 study LGAs was used for the study. The study participants were Household Heads (HHs), selected through multistage systematic random sampling technique. The head of each of the selected household was enrolled into the study for oral interview, questionnaire administration, and blood sample collection. The sampling of participants was carried out in the Primary Health Centres (PHCs) in the study LGAs.
The informed consent of selected HHs willing to participate in the study was obtained prior to enrolment into the study. The inclusion criteria used in the enrolment of participants for the study was based on location of the participant household within the study sampling frames, consent to participate and willingness to comply with study requirements and procedures, irrespective of showing symptoms of uncomplicated malaria or mild fever. Individual who does not reside within the sampling frame, or is severely ill, showing gross symptoms of severe diseases like severe malaria or refused to give their informed consent, were excluded from participating in the study.
Oral interview and questionnaire administration
Pretested and structured questionnaires were administered by an already oriented and trained volunteer medical recorder to each of the 360 selected HHs. Each questionnaire was sought into 3 categories of information, which included; socio-demographic characteristics of respondents and the household members, which includes age, sex, marital status, occupation, education and income level; mosquito-prevention practices and knowledge about mosquito breeding; and lastly, environmental and behavioural factors of vulnerability to malaria which included presence of: temporary pool (stagnant) of water, gutter for draining used water, thick surrounding vegetation, uncovered bowls and drums filled with water, fish pond/open reservoirs, refuse/waste dump site, window and/or door nets and open septic tanks and/or bathing/ used water storage pits. The correctness of chosen environmental and behavioural risk factor options, were ascertained through visits to and visual inspection of participants’ residences.
Blood samples were collected using a 2.5 ml syringe by a volunteer health worker in each of the study PHC. The collected blood samples (0.25 ml to 0.5 ml) were immediately transferred into labelled heparinised sample bottles, which were stored in refrigerator for further analysis.
Microscopic procedures were done according to guidelines by [16]. A plastic pipette was used to collect blood sample from the labelled heparinized sample bottle and a single small drop of blood was gently put on the middle of a labelled microscope slide for thin film. The thin film was examined under the microscope at 100x magnification to check for the presence and confirmation of malaria parasite species. A different and experienced laboratory technician also examined the slides to ensure quality assurance [17].
Results
The prevalence of malaria was 32.2%. Participants above 60 years were at highest risk of having malaria, O.R=1.35, C.I (0.29-6.21) relative to participants between 0 to 15 years. The odds ratios of participants in relation to educational level using no formal education as the reference level were O.R=0.462, C.I (0.06-3.64); O.R=2.517, C.I (0.83-7.67); and O.R=0.806, C.I (0.42-1.55) for primary, secondary and tertiary education levels respectively. The odds of having malaria among trained employees, traders, civil servants, and pensioners were O.R=2.125, C.I (0.41-11.12), O.R=1.836, C.I (0.36-9.41), O.R=2.636, C.I (0.46-15.09) and O.R=3.000, (C.I=0.19-47.96) respectively in relation to unemployed participants.
Participants without a previous malaria episode within the last one year prior to the conduct of the study were at relatively higher odds of having malaria, O.R=1.33, (C.I=0.86-2.08), relative to those who did not have a previous episode. Participants who sought treatment in designated healthcare facilities were least likely to have malaria, O.R=0.000, C.I (0.000) relative to those who sought treatment in inappropriate places. Participants who are not aware of malaria as a disease were 2.113 times more likely to have malaria, O.R=2.11, CI (0.13-34.08). A total of two hundred and eight (208), which accounted for 57.8% of the participants own at least one bed net (Figure 1), out of which only 8 (4%), claimed to sleep under it at night. The prevalence of malaria was 33.7% among participants who possess bed nets compared with those who do not (30.3%), X̅2 (1, N=360) =1.81, p=0.50.
Malaria prevalence was relatively higher among participants who do not know at least one standard mosquito prevention practice (33.2%) compared with those who knew (30.3%), X ̅2 (1, N=360) =1.55, p=0.57 (Figure 2). Malaria prevalence was 28.8% among participants who had stagnant pools in their surrounding compared with 33.9% observed among participants who don’t have (Figure 3), X ̅2 (1, N=360) =1.17, p=0.33. Sixty-three percent of the participants do not have closed windows and or door nets (Figure 4). Malaria prevalence was insignificantly higher among participants with closed windows and or door nets (32.4%) compared with participants who do not have (32.1), X ̅2(1, N=360) =2.33, p=0.95.
Discussion
Bello and Hassan (2017) had previously reported a slightly higher prevalence (36.1%) in Epe. The long term regular use of malaria control interventions has been found to reduce malaria incidence. The decline in prevalence in this study may be attributed to the impact of malaria control interventions used since the past years. Malaria prevalence has long been established to be age dependent [17], with older children (>5 yrs) having higher tendency of having the disease, possibly due to relatively reduced intervention rate, late sleeping habit and increased attractiveness to mosquitoes compared with children below the age of 5 years [18-20]. This may explain why the odd of having malaria was highest among participants aged 60 years. The impact of good knowledge about mosquito prevention practices and adoption of one or more of the practices on malaria incidence [21,22] cannot be over-emphasized in this study. A study showed that individuals, who have mosquito nets in their rooms, have less chance of having household members testing positive for RDT [23]. However, high malaria prevalence observed among those who own a bed net may imply that owning a bed net may not necessarily translate to usage due to earlier reported factors such as Heat, low mosquito activity, phobia for chemicals, lack of space and difficulty in hanging nets, preference for other preventive measures, ignorance and cultural beliefs [24].
Several studies have hypothesized that environmental features and behavioural factors of vulnerability to malaria may be significant risk factors for malaria [25-27]. This may justify the low malaria prevalence observed among participants having good knowledge about one or more standard mosquito prevention practices, implying that knowing and adopting one or more standard mosquito prevention practices is likely to reduce malaria incidence.
Analysis showed that there was spatial correlation of malaria incidence with environmental factors in India. Villages under malaria hotspots are characterised with abundant water bodies. However, this was contrary to this study as malaria prevalence was lower among participants who had stagnant pools and relatively higher among those having window/door net in their homes. This may imply that other risk factors such as use of personal protective measures such as sleeping under bed nets are crucial in malaria transmission.
Conclusion
Sociodemographic, behavioural and environmental risk factors, such as age, educational level, occupation, previous malaria episode, treatment facility, malaria awareness and preventability, have varying influence on the occurrence of malaria. Seeking treatment at designated healthcare facilities significantly reduced malaria incidence. However, owning mosquito bed nets, possessing window/door nets and absence of surrounding puddles do not significantly reduce the incidence of malaria.
There is therefore the need to sensitize with the people to not only get treatment at designated healthcare facilities but also adopt the practice of using personal protections such as bed net usage, closing of window/door nets especially at night.
Competing Interests
The authors declare that they have no competing interests.
References
- Dawaki S, Al-Mekhlafi HM, Ithoi I, Ibrahim J, Atroosh WM, Abdulsalam AM, et al. Is Nigeria winning the battle against malaria? Prevalence, risk factors and KAP assessment amonog Hausa communities in Kano State. Malar J 2016;15:351.
[Crossref] [Google scholar] [Pubmed]
- Nwaneli EI, Eguonu I, Ebenebe JC, Osuorah CDI, Ofiaeli OC, Nri-Ezedi CA. Malaria prevalence and its sociodemographic determinants in febrile children–a hospital based study in a developing community in South-East Nigeria. J Prev Med Hyg 2020;61:e173-e180.
[Crossref] [Google scholar] [Pubmed]
- Ernst KC, Lindblade KA, Koech D, Sumba PO, Kuwuor DO, et al. Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands: a case-control study. Trop Med Int Healt 2009;14:1258-1265.
[Crossref] [Google scholar] [Pubmed]
- Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci USA 2006;103:5829-5834.
[Crossref] [Google scholar] [Pubmed]
- Peterson I, Borrell LN, El-Sadr W, Teklehaimanot A. Individual and household level factors associated with malaria incidence in a highland region of Ethiopia: a multilevel analysis. Am J Trop Med Hyg 2009; 80:103-111.
[Crossref] [Google scholar] [Pubmed]
- Jamison DT, Mosley WH, Measham AR, Bobadilla A. Disease control priorities in Developing countries. The World Bank 1993.
- Ojiezeh TI, Ibeh NI, Opedun DO, Udoh SJ. Malaria Endemicity among Pregnant Women in Urban and Semi-Urban Areas in Southwest, Nigeria. American-Eurasian Journal of Scientific Research 2010; 5:207-211.
- Tolulope O. Spatio-Temporal Clustering of Malaria Morbidity in Nigeria (2004-2008). Journal of Science Research 2014;13:99-113.
- Adepoju KA, Akpan GE. Historical Assessment of Malaria Hazard and Mortality in Nigeria- Cases and Deaths: 1955-2015. International Journal of Environmental Bioenergy 2017; 121:30-46.
- Torr SJ, Mangwiro TN, Hall DR. The effects of host physiology on the attraction of tsetse (Diptera: Glossinidae) and Stomoxys (Diptera: Muscidae) to cattle. Bull Entomol Res 2006; 96: 71-84.
[Crossref] [Google scholar] [Pubmed]
- Havlicek J, Roberts SC, Flegr J. Women's preference for dominant male odour: effects of menstrual cycle and relationship status. Biol Lett 2005;13:256-259.
[Crossref] [Google scholar] [Pubmed]
- Mohammed US, Iyiola AS, Usman RK. Production analysis of catfish farming in Epe Local Government Area of Lagos State. Int J Agri Tech 2015;11:153-161.
- Hassan B. A study of inland waterways transport in Epe area of Lagos 2012.
- AIRS. Nigeria Final Entomology Report. Africa Indoor Residual Spraying Project 2015.
- Odugbemi BA, Wright KO, Onajole AT, Kuyinu YA, Goodman OO, Odugbemi TO. A malariometric survey of under‑fives residing in indoor residual spraying‑implementing and non‑implementing communities of Lagos, Nigeria. Malaria Journal 2016; 15:458.
[Crossref] [Google scholar] [Pubmed]
- WHO. Basic malaria microscopy, Part I. Learner’s guide. 2010.
- WHO. Malaria microscopy quality assurance manual. 2009.
- Thomson MC, Connor SJ, Milligan PJ. Flasse SP. The ecology of malaria as seen from earth observation satellites. Ann Tropi Med Parasitol. 1996; 90:243-264.
[Crossref] [Google scholar] [Pubmed]
- Ajegena BK, Oti VB. The Challenges of Using Insecticides Treated Nets (ITNs) in Curbing Malaria in Nigeria: A 2000-2018 Systematic Review. J Infect Dis Epidemiol 2020;6:140.
- Aina OO, Agomo CO, Olukosi YA, Okoh HI, Iwalokun BA, Egbuna KM, et al. Malariometric Survey of Ibeshe Community in Ikorodu, Lagos State: Dry Season. Malar Res Treat 2013;487250.
[Crossref] [Google scholar] [Pubmed]
- Koram KA, Bennett S, Adiamah JH, Greenwood BM. Socioeconomic risk-factors for malaria in a periurban area of the Gambia. Trans R Soc Trop Med Hyg 1995;89:146-150.
[Crossref] [Google scholar] [Pubmed]
- Prevalence, risk factors and KAP assessment amonog Hausa communities in Kano State
- Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet 2000;355:1972.
[Crossref] [Google scholar] [Pubmed]
- Nwajide CS. Geology of Nigeria's Sedimentary Basins. 2013. Lagos: CSS Bookshops Ltd.
- Okorie FC. A spatio-temporal analysis of deforestation in Epe and its environs, Lagos, Nigeria. International Journal of Science Environment and Technology 2012;1:548–562. [Crossref]
- Rejmankova E, Roberts DR, Pawley A, Manguin S, Polanco J. Predictions of adult Anopheles albimanus densities in villages based on distances to remotely sensed larval habitats. Am J Trop Med Hyg 1995;53:482–488.
[Crossref] [Google scholar] [Pubmed]
- Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, et al. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg 1992;47:181-189.
[Crossref] [Google scholar] [Pubmed]
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.







 ) Positive, (
) Positive, (  ) Negative)
) Negative)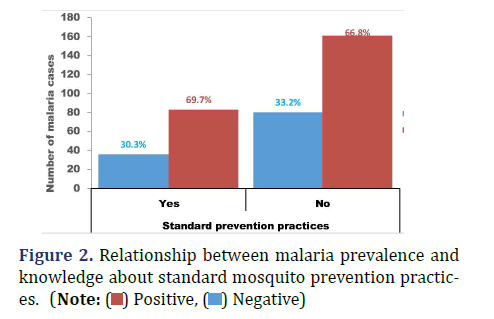
 ) Positive, (
) Positive, (  ) Negative)
) Negative)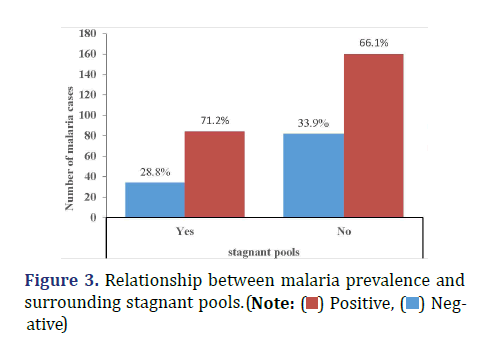
 ) Positive, (
) Positive, (  ) Negative)
) Negative)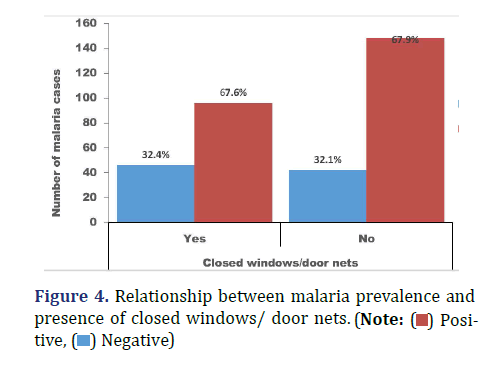
 ) Positive, (
) Positive, ( ) Negative)
) Negative)